
The pharmaceutical industry operates under one fundamental principle: ensuring that the benefits of medicines outweigh their risks. This critical balance is maintained through pharmacovigilance – a discipline that has evolved significantly over the past decades and continues to shape how we develop, monitor, and manage pharmaceutical products in 2025.
For pharmaceutical leaders, understanding pharmacovigilance is not just about regulatory compliance; it’s about safeguarding public health while maintaining the trust that enables innovation and growth in our industry. This guide provides a comprehensive overview of pharmacovigilance essentials for today’s pharmaceutical executives.
Pharmacovigilance, often termed PV, is the science and practice of monitoring, assessing, understanding, and preventing adverse effects or other drug-related problems. The World Health Organization (WHO) defines it as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem.”
The primary goals of pharmacovigilance extend beyond simple adverse event reporting. Modern PV systems aim to:
Pharmacovigilance has transformed dramatically since its formal establishment following the thalidomide tragedy of the 1960s. Today’s PV landscape incorporates advanced technologies, real-world evidence, and proactive risk management strategies that were unimaginable decades ago.
In 2025, we see pharmacovigilance embracing artificial intelligence, machine learning, and big data analytics to enhance signal detection and risk assessment capabilities. This evolution reflects our industry’s commitment to continuous improvement in patient safety.
The regulatory environment governing pharmacovigilance continues to evolve, with agencies worldwide implementing more sophisticated approaches to drug safety monitoring. Understanding these requirements is crucial for pharmaceutical leaders operating in global markets.
The U.S. Food and Drug Administration maintains comprehensive pharmacovigilance requirements through the FDA Adverse Event Reporting System (FAERS) and Risk Evaluation and Mitigation Strategies (REMS). Companies must submit periodic safety reports and maintain robust safety databases.
The European Medicines Agency operates under the EU pharmacovigilance legislation, requiring detailed Periodic Safety Update Reports (PSURs) and Risk Management Plans (RMPs). The EudraVigilance database serves as the central repository for adverse event reports across EU member states.
The International Council for Harmonisation (ICH) continues to provide globally accepted standards, with ICH E2A through E2F guidelines forming the foundation of international pharmacovigilance practices.
Regulatory agencies have increasingly focused on real-world evidence integration, digital health technologies, and patient-centric approaches to safety monitoring. These developments require pharmaceutical companies to adapt their pharmacovigilance strategies accordingly.
Modern pharmaceutical companies face complex compliance challenges, including managing global reporting timelines, ensuring data quality across multiple systems, and adapting to evolving regulatory expectations. Successful organizations invest in robust quality management systems and maintain close relationships with regulatory authorities.
An effective pharmacovigilance system comprises several interconnected components that work together to ensure comprehensive safety monitoring throughout a product’s lifecycle.
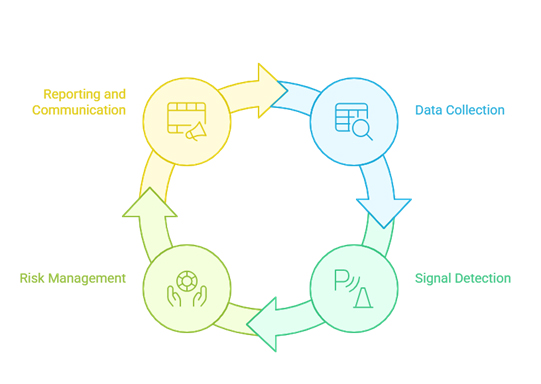
The foundation of any pharmacovigilance system is the systematic collection of adverse event reports from multiple sources, including healthcare professionals, patients, clinical trials, and literature reviews. Modern systems must handle increasing volumes of data from diverse sources while maintaining data quality and integrity.
Contemporary pharmacovigilance systems integrate data from various sources:
Signal detection involves identifying potential safety concerns from large datasets of adverse event reports. Advanced analytics and machine learning algorithms increasingly supplement traditional statistical methods to identify patterns that might indicate new safety risks.
Traditional signal detection relies on disproportionality analysis, comparing the reporting rates of specific drug-event combinations against background rates. Modern approaches incorporate temporal analysis, medical confirmation, and multi-dimensional assessment criteria.
Qualified medical professionals evaluate detected signals to determine clinical significance and establish causal relationships between drugs and adverse events. This process requires expertise in pharmacology, epidemiology, and clinical medicine.
Comprehensive risk management plans outline identified risks, potential risks, and missing information about a pharmaceutical product. These plans detail specific activities to characterize and minimize risks while ensuring appropriate benefit-risk balance.
When safety concerns are identified, companies implement various risk minimization strategies, including:
Pharmaceutical companies must submit various types of safety reports to regulatory authorities, including Individual Case Safety Reports (ICSRs), Periodic Safety Update Reports (PSURs), and Development Safety Update Reports (DSURs).
Effective pharmacovigilance systems maintain clear internal communication channels to ensure that safety information reaches appropriate stakeholders, including medical affairs, clinical development, and senior management teams.
The integration of advanced technologies has revolutionized pharmacovigilance practices, enabling more efficient and effective safety monitoring in 2025.
AI and machine learning algorithms can process vast amounts of safety data to identify potential signals more quickly and accurately than traditional methods. These technologies can detect complex patterns and relationships that might be missed by conventional statistical approaches.
NLP technologies help extract relevant safety information from unstructured data sources, including medical literature, social media posts, and free-text sections of adverse event reports. This capability significantly expands the scope of safety monitoring.
Access to electronic health records provides unprecedented insights into real-world drug safety and effectiveness. This data source enables more comprehensive safety assessments and supports regulatory decision-making.
Insurance claims databases offer valuable information about drug utilization patterns and associated healthcare outcomes, supporting pharmacoepidemiological studies and safety signal validation.
Consumer health technologies generate continuous streams of health data that can provide early indicators of adverse drug reactions. Integration of this data into pharmacovigilance systems represents a significant opportunity for enhanced safety monitoring.
Digital platforms enable direct collection of patient-reported adverse events and outcomes, providing patient perspectives that complement healthcare professional reporting.
Developing a comprehensive pharmacovigilance strategy requires careful planning, adequate resources, and strong organizational commitment to patient safety.
The QPPV serves as the single point of contact for pharmacovigilance matters and bears overall responsibility for the pharmacovigilance system. This role requires specific qualifications and cannot be delegated to others.
The PSMF documents how an organization conducts pharmacovigilance activities and serves as the primary reference for regulatory inspections. Maintaining an up-to-date PSMF is essential for regulatory compliance.
Effective pharmacovigilance requires skilled professionals with expertise in medicine, pharmacy, epidemiology, and regulatory affairs. Organizations must invest in training and development to maintain competent teams.
Modern pharmacovigilance systems require robust technology infrastructure capable of handling large volumes of data while maintaining security and regulatory compliance.
Comprehensive SOPs ensure consistent and compliant pharmacovigilance practices across all organizational levels. These procedures must be regularly updated to reflect evolving regulatory requirements and best practices.
Regular training programs ensure that all staff members understand their roles and responsibilities in pharmacovigilance activities. Competency assessments help identify areas for improvement and ensure consistent performance.
Different pharmaceutical products present varying levels of safety risk based on their therapeutic class, patient population, and known safety profile. Risk-based approaches allow organizations to allocate resources appropriately.
Effective pharmacovigilance strategies include mechanisms for continuous monitoring and adaptation based on emerging safety data and changing regulatory requirements.
Many pharmaceutical companies partner with specialized service providers for certain pharmacovigilance activities. Successful outsourcing requires careful vendor selection, clear contractual agreements, and ongoing oversight to ensure quality and compliance.
When selecting pharmacovigilance service providers, consider factors such as:
Effective oversight of outsourced pharmacovigilance activities requires regular audits, performance monitoring, and clear communication channels between the pharmaceutical company and service provider.
Pharmacovigilance has become an indispensable pillar of modern pharmaceutical operations, evolving into a highly strategic function driven by advanced technologies, regulatory demands, and a growing focus on patient-centric care. As we move through 2025, pharma leaders must go beyond compliance—embracing innovation, real-world evidence, and intelligent systems to safeguard public health. Building a robust, adaptive pharmacovigilance framework is not only essential for maintaining regulatory alignment but also for earning trust, ensuring drug safety, and supporting long-term organizational success in an increasingly complex global market.