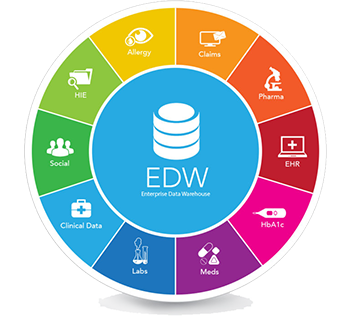
Clinical Data Warehouse and Analytics
Life Sciences organizations constantly face the challenge of building a flexible and scalable platform which can consume, aggregate, transform and enrich the data, and that can be accessed through a variety of tools to enable full data exploration.
Vitrana‘s Clinical IT Services team has extensive expertise in designing, deploying, implementing and managing full blown Clinical Development Analytics environment for the aggregation, analysis and reporting of Clinical data from multiple source systems.
Vitrana‘s expert team have credible and demonstrated experience in the implementation of the following Health Sciences Applications for multiple Life Sciences organizations globally:
- BioClinica StudyView, Medidata OPAL, Oracle Clinical Development Analytics (CDA)
- Bioclinica RBM, Medidata RBM
- Oracle Life Sciences Data Hub (LSH), Oracle Health Sciences Data Management Workbench (DMW)