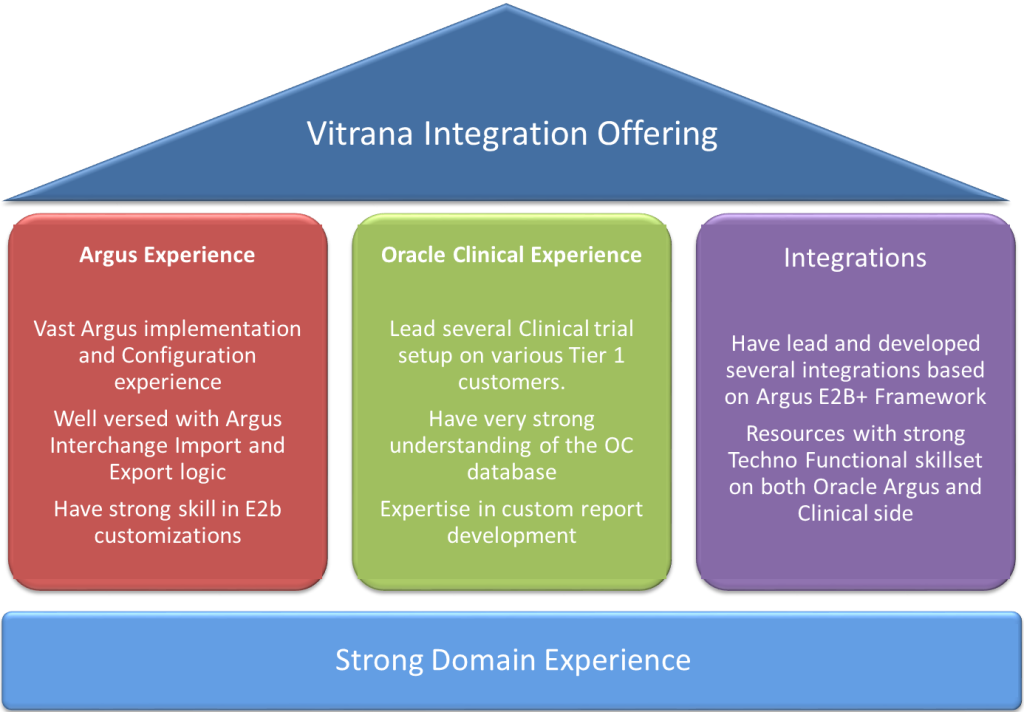
Overview
Today’s Life Sciences organizations face significant challenges in improving the quality of clinical services and meeting their regulatory compliance obligations. The success of life sciences organizations is predicated on many factors, but one that is particularly critical concerns the ability to fully leverage information assets to maximum effect. The proactive and efficient processing of real-time information across the enterprise can provide up-to-the-minute, analytical insights that help an organization to make effective and informed decisions. In addition, effective sharing of information across business applications enhances both internal efficiency and quality of service for internal stakeholders and customers alike. However, more often than not, these information assets lie hidden within the individual applications, preventing organizations from realizing their true potential.