The pharmaceutical industry stands at a transformative crossroads. As drug safety monitoring becomes increasingly complex, artificial intelligence is emerging as a powerful ally in pharmacovigilance (PV) – the science of detecting, assessing, and preventing adverse drug reactions. For industry professionals navigating this evolving landscape, understanding AI’s role in pharmacovigilance isn’t just beneficial; it’s becoming essential.
Every day, millions of patients worldwide take medications, generating vast amounts of safety data across clinical trials, medical records, social media, and regulatory databases. Traditional pharmacovigilance methods, while foundational, are struggling to keep pace with this data explosion. Enter artificial intelligence – a technology that promises to revolutionize how we monitor drug safety, detect signals, and protect patients.
This guide will demystify AI in pharmacovigilance, exploring its current applications, benefits, challenges, and future potential. Whether you’re a pharmaceutical executive, regulatory affairs professional, or safety scientist, this comprehensive overview will equip you with the knowledge needed to navigate the AI-driven future of drug safety monitoring.
2. Understanding Pharmacovigilance in the Modern Era
2.1 The Foundation of Drug Safety
Pharmacovigilance encompasses all activities related to the detection, assessment, understanding, and prevention of adverse drug reactions and other drug-related problems. At its core, PV ensures that the benefits of medications continue to outweigh their risks throughout their lifecycle – from clinical development through post-market surveillance.
Traditional pharmacovigilance relies heavily on spontaneous reporting systems, where healthcare providers, patients, and pharmaceutical companies report suspected adverse events to regulatory authorities. These reports are then manually reviewed, coded, and analyzed to identify potential safety signals.
2.2 Current Challenges in Pharmacovigilance
The pharmaceutical industry faces several mounting challenges in drug safety monitoring:
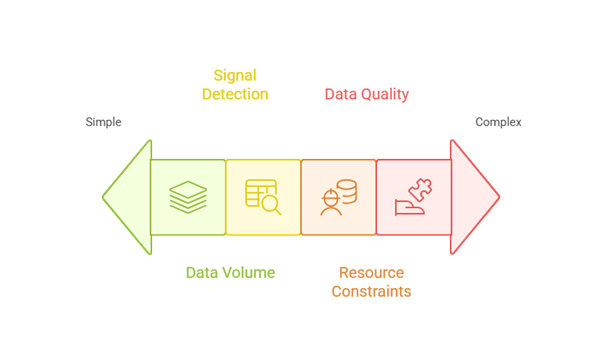
Data Volume and Complexity: The sheer volume of safety data has grown exponentially. Regulatory databases now contain millions of adverse event reports, with thousands added daily. This creates significant strain on traditional manual processing systems.
Signal Detection Delays: Manual review processes can delay the identification of important safety signals, potentially putting patients at risk. The time between initial adverse event occurrence and regulatory action can span months or even years.
Resource Constraints: Pharmacovigilance departments require skilled professionals to review cases, assess causality, and generate reports. The specialized nature of this work, combined with increasing data volumes, creates ongoing resource pressures.
Data Quality Issues: Inconsistent reporting standards, incomplete information, and varying data formats across different sources complicate analysis and decision-making.
2.3 The Digital Transformation Imperative
These challenges have created an urgent need for digital transformation in pharmacovigilance. Organizations are increasingly recognizing that manual processes alone cannot handle the scale and complexity of modern drug safety monitoring. This realization has paved the way for AI adoption across the pharmaceutical industry.
3. AI Technologies Transforming Pharmacovigilance
3.1 Natural Language Processing: Understanding Unstructured Data
Natural Language Processing (NLP) represents one of the most impactful AI applications in pharmacovigilance. Since approximately 80% of pharmacovigilance data exists in unstructured formats – including medical narratives, clinical notes, and literature reports – NLP technology is crucial for extracting meaningful information.
Key NLP Applications:
- Automated Case Processing: NLP systems can automatically extract relevant information from medical narratives, including patient demographics, medical history, drug details, and adverse event descriptions
- Medical Literature Monitoring: AI can scan thousands of medical publications daily, identifying potential safety signals and relevant case reports
- Social Media Surveillance: Advanced NLP algorithms monitor social media platforms for patient-reported adverse events and drug experiences
3.2 Machine Learning: Pattern Recognition and Prediction
Machine learning algorithms excel at identifying patterns in large datasets that might escape human detection. In pharmacovigilance, these systems can:
- Signal Detection: ML algorithms analyze vast databases to identify statistical associations between drugs and adverse events, potentially detecting safety signals months before traditional methods.
- Risk Prediction: Predictive models can assess the likelihood of adverse events based on patient characteristics, drug combinations, and historical data.
- Case Prioritization: ML systems can automatically prioritize cases based on severity, regulatory requirements, and potential impact, ensuring that critical cases receive immediate attention.
3.3 Deep Learning: Advanced Pattern Recognition
Deep learning, a subset of machine learning, uses neural networks to process complex data patterns. In pharmacovigilance, deep learning applications include:
- Image Analysis: Processing medical images to identify drug-related skin reactions or other visual adverse events
- Complex Data Integration: Combining multiple data sources (clinical, genetic, demographic) to provide comprehensive safety assessments
- Temporal Pattern Recognition: Identifying time-dependent relationships between drug exposure and adverse events
4. Current Applications and Use Cases
4.1 Automated Adverse Event Processing
Leading pharmaceutical companies are implementing AI-powered systems to streamline adverse event processing. These systems can:
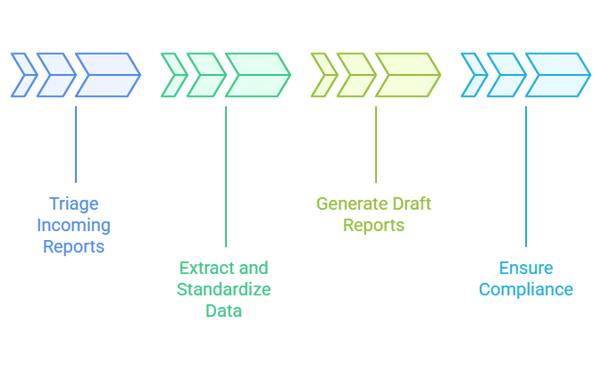
- Automatically triage incoming reports based on severity and regulatory requirements
- Extract and standardize information from diverse data sources
- Generate draft case reports for human review and validation
- Ensure compliance with regulatory timelines and requirements
Real-World Example: A major pharmaceutical company implemented an AI system that reduced case processing time by 60% while improving data quality and consistency. The system automatically extracts key information from medical narratives, suggests appropriate medical coding, and flags cases requiring urgent attention.
4.2 Enhanced Signal Detection
Traditional signal detection methods rely on statistical measures like disproportionality analysis. AI enhances these approaches by:
- Integrating multiple data sources simultaneously
- Identifying subtle patterns that traditional methods might miss
- Reducing false positive signals through advanced filtering algorithms
- Providing context for statistical associations
Case Study: A regulatory agency implemented an AI-powered signal detection system that combines spontaneous reporting data with electronic health records and claims databases. The system identified a previously unknown drug-drug interaction three months earlier than traditional methods would have detected it.
4.3 Literature and Social Media Monitoring
AI systems now continuously monitor medical literature and social media platforms for safety-related information:
Literature Surveillance: AI can process thousands of medical publications daily, identifying relevant adverse event reports and extracting key safety information. This capability is particularly valuable for post-market surveillance of established drugs.
Social Media Analysis: Advanced NLP systems monitor platforms like Twitter, Reddit, and patient forums for adverse event reports and patient experiences. While these sources require careful validation, they can provide early warning signals and insights into real-world drug use patterns.
4.4 Regulatory Compliance and Reporting
AI assists with regulatory compliance by:
- Automating report generation for regulatory submissions
- Ensuring consistency in medical coding and case classification
- Tracking regulatory timelines and flagging approaching deadlines
- Standardizing data formats across different regulatory jurisdictions
5. Benefits and Advantages of AI in Pharmacovigilance
5.1 Enhanced Efficiency and Speed
AI transforms pharmacovigilance operations through dramatic improvements in processing speed and efficiency:
- Faster Case Processing: AI systems can process adverse event reports in minutes rather than hours, significantly reducing turnaround times for regulatory submissions.
- 24/7 Monitoring: Unlike human reviewers, AI systems can continuously monitor data sources, providing real-time surveillance of drug safety signals.
- Scalability: AI systems can handle increasing data volumes without proportional increases in human resources.
5.2 Improved Data Quality and Consistency
AI contributes to better data quality through:
- Standardized Processing: AI systems apply consistent rules and criteria across all cases, reducing variability in data interpretation and coding.
- Error Reduction: Automated systems eliminate common human errors such as transcription mistakes and coding inconsistencies.
- Comprehensive Data Capture: AI can extract information from sources that might be overlooked in manual reviews, ensuring more complete safety assessments.
5.3 Enhanced Signal Detection Capabilities
AI-powered signal detection offers several advantages over traditional methods:
- Multi-dimensional Analysis: AI can simultaneously analyze multiple variables and data sources
- Pattern Recognition: Advanced algorithms can identify subtle patterns that might escape human detection
- Reduced False Positives: Sophisticated filtering algorithms help focus attention on truly significant signals
- Contextual Understanding: AI can provide additional context for statistical associations, improving signal assessment
5.4 Cost Reduction and Resource Optimization
Organizations implementing AI in pharmacovigilance typically experience:
- Reduced Labor Costs: Automation of routine tasks allows human experts to focus on high-value activities requiring clinical judgment.
- Faster Time to Market: More efficient safety monitoring can reduce regulatory review times and accelerate drug approvals.
- Improved Compliance: Automated systems help ensure consistent compliance with regulatory requirements, reducing the risk of penalties and delays.
6. Challenges and Limitations
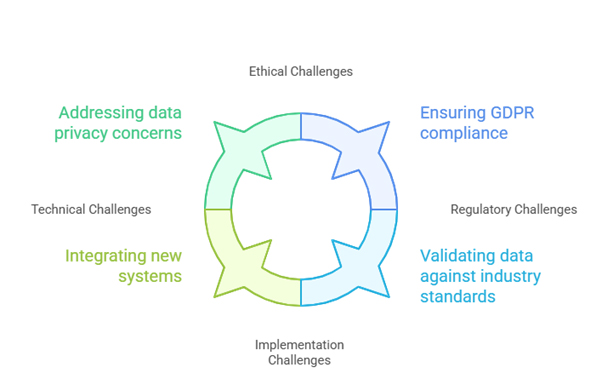
6.1 Data Quality and Standardization Issues
Despite AI’s capabilities, data quality remains a fundamental challenge:
- Inconsistent Data Sources: Different databases, reporting systems, and jurisdictions use varying data formats and standards, complicating AI implementation.
- Missing Information: Adverse event reports often lack complete information, requiring AI systems to make inferences or flag cases for human review.
- Biased Training Data: AI models are only as good as the data they’re trained on. Historical biases in reporting patterns can perpetuate or amplify existing problems.
6.2 Regulatory Acceptance and Validation
The regulatory landscape for AI in pharmacovigilance is still evolving:
- Validation Requirements: Regulatory agencies require extensive validation of AI systems before accepting their outputs for decision-making.
- Transparency and Explainability: AI systems must be able to explain their reasoning and decisions, particularly for regulatory submissions.
- Changing Guidelines: Regulatory guidance for AI in pharmacovigilance continues to evolve, requiring ongoing adaptation of systems and processes.
6.3 Technical and Implementation Challenges
Organizations face several technical hurdles when implementing AI:
- Integration Complexity: Incorporating AI into existing pharmacovigilance systems requires significant technical expertise and resources.
- Change Management: Staff must be trained on new AI-powered workflows and systems, requiring substantial change management efforts.
- Maintenance and Updates: AI systems require ongoing maintenance, updates, and retraining to maintain effectiveness.
6.4 Ethical and Privacy Considerations
AI implementation in pharmacovigilance raises important ethical questions:
- Patient Privacy: AI systems must comply with strict privacy regulations while accessing and analyzing patient data.
- Algorithmic Bias: AI systems may perpetuate or amplify existing biases in healthcare data, potentially affecting certain patient populations unfairly.
- Decision-Making Transparency: Organizations must ensure that AI-driven decisions can be explained and justified to regulators and stakeholders.
7. Future Outlook and Emerging Trends
7.1 Integration with Real-World Evidence
The future of AI in pharmacovigilance will likely involve deeper integration with real-world evidence (RWE) sources:
- Electronic Health Records: AI systems will increasingly incorporate EHR data to provide comprehensive safety monitoring across entire patient populations.
- Wearable Technology: Data from fitness trackers, smartwatches, and medical devices will provide continuous monitoring of patient health and potential adverse events.
- Genomic Data: Integration of genetic information will enable personalized risk assessment and targeted safety monitoring.
7.2 Advanced Predictive Analytics
Future AI systems will move beyond reactive signal detection to predictive safety monitoring:
- Risk Stratification: AI will identify high-risk patient populations before adverse events occur, enabling proactive interventions.
- Personalized Safety Profiles: Individual patient risk assessments will be based on genetic factors, medical history, and lifestyle data.
- Preventive Measures: AI-driven insights will inform the development of risk mitigation strategies and patient education programs.
7.3 Regulatory Evolution
The regulatory landscape will continue adapting to AI capabilities:
- Standardized Guidelines: Regulatory agencies will develop comprehensive guidance for AI validation and implementation in pharmacovigilance.
- Automated Reporting: Direct integration between AI systems and regulatory databases will streamline reporting processes.
- Global Harmonization: International cooperation will drive standardization of AI approaches across different regulatory jurisdictions.
7.4 Collaborative Platforms
The future will likely see increased collaboration through AI-powered platforms:
- Industry Consortiums: Pharmaceutical companies will share anonymized safety data to improve AI model performance across the industry.
- Academic Partnerships: Collaboration with academic institutions will drive innovation in AI methodologies and applications.
- Regulatory Partnerships: Public-private partnerships will accelerate the development and validation of AI systems for pharmacovigilance.
8. Conclusion
Artificial intelligence is redefining pharmacovigilance, offering transformative solutions to long-standing challenges in drug safety monitoring. From automating case processing to uncovering patterns in vast datasets, AI enables faster, more accurate, and more scalable pharmacovigilance operations. As the pharmaceutical landscape grows more complex, AI equips organizations to not only keep pace but to lead in ensuring patient safety.
However, successful adoption goes beyond technology—it requires a thoughtful, strategic approach that considers regulatory frameworks, data quality, integration challenges, and ethical responsibilities. Organizations that take the initiative to evaluate their current systems, invest in the right tools, and upskill their teams will be better positioned to navigate the evolving landscape and deliver on their commitment to patient care.
The question is no longer if AI should be adopted in pharmacovigilance, but how and when. For those ready to begin, the time is now.



