
The pharmaceutical industry sits at a crossroads of innovation and responsibility. While companies race to develop life-saving treatments, they must simultaneously ensure these medications remain safe for millions of patients worldwide. Enter pharmacovigilance — the science of monitoring drug safety throughout a medication’s lifecycle.
Today, artificial intelligence is revolutionizing this critical field. From processing thousands of adverse event reports in minutes to predicting potential safety signals before they become widespread issues, AI is transforming how pharmaceutical companies protect patient safety. This technological shift isn’t just about efficiency; it’s about saving lives through faster, more accurate safety monitoring.
For pharmaceutical leaders, understanding AI’s role in pharmacovigilance has become essential. Companies that embrace these technologies are not only improving patient outcomes but also gaining competitive advantages in regulatory compliance, risk management, and operational efficiency. This guide explores how industry leaders are leveraging AI to enhance pharmacovigilance and what it means for the future of drug safety.
Pharmacovigilance (PV) is the systematic monitoring of adverse drug reactions and other medicine-related problems. It encompasses the entire process of collecting, analyzing, and responding to safety information about pharmaceutical products. This includes everything from clinical trial data to post-market surveillance reports from healthcare providers and patients.
Traditional pharmacovigilance relies heavily on manual processes. Safety teams review individual case safety reports (ICSRs), analyze patterns, and prepare regulatory submissions. While effective, these manual approaches face significant challenges in today’s pharmaceutical landscape.
The volume of safety data has exploded in recent years. Regulatory authorities worldwide receive millions of adverse event reports annually, and this number continues to grow. Social media platforms, patient forums, and digital health applications generate additional streams of safety-relevant information that traditional systems struggle to process.
Meanwhile, regulatory expectations have intensified. Agencies like the FDA and EMA require faster reporting timelines and more comprehensive safety analyses. Companies must identify potential safety signals earlier and respond more quickly to emerging risks.
AI addresses these challenges through several key capabilities:
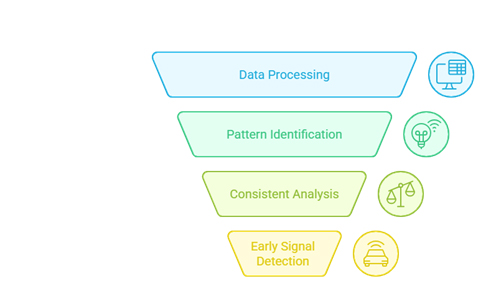
Speed and Scale: AI systems can process thousands of safety reports in the time it takes humans to review dozens. Natural language processing algorithms can extract relevant information from unstructured text, while machine learning models can identify patterns across vast datasets.
Consistency: Human reviewers may interpret the same safety report differently. AI systems provide consistent analysis, reducing variability in safety assessments and improving regulatory compliance.
Early Detection: Advanced AI models can identify potential safety signals before they become apparent through traditional statistical methods. This early warning capability enables proactive risk management.
Cost Efficiency: While initial implementation requires investment, AI systems ultimately reduce the human resources needed for routine pharmacovigilance tasks, allowing safety teams to focus on complex analyses and strategic decisions.
One of the most immediate applications of AI in pharmacovigilance is automated case processing. Traditional methods require safety professionals to manually review each adverse event report, extract relevant information, and determine whether it represents a valid safety concern.
AI-powered systems can now automate much of this process. Natural language processing algorithms read through medical narratives, identify key information like patient demographics, medications, and adverse events, and classify reports according to regulatory standards. For example, these systems can automatically determine whether a report meets the criteria for an Individual Case Safety Report (ICSR) and flag cases that require immediate attention.
Real-World Impact: A major pharmaceutical company recently implemented an AI system that reduced case processing time by 70%. The system automatically extracts structured data from incoming safety reports, allowing safety teams to focus on complex cases requiring human expertise.
Signal detection—identifying new or changing safety patterns—represents another area where AI excels. Traditional pharmacovigilance relies on statistical methods that may miss subtle patterns or require large numbers of cases before detecting a signal.
Machine learning algorithms can analyze multiple data sources simultaneously, identifying correlations that might escape human notice. These systems can detect unusual patterns in adverse event reporting, changes in the severity or frequency of known side effects, or emerging safety concerns in specific patient populations.
Advanced Capabilities: Some AI systems combine structured safety data with unstructured information from social media, patient forums, and medical literature. This comprehensive approach provides a more complete picture of a drug’s safety profile in real-world settings.
Keeping up with medical literature is crucial for comprehensive pharmacovigilance. Safety teams must monitor thousands of scientific publications, conference abstracts, and regulatory communications for relevant safety information.
AI systems can automate literature surveillance by scanning publications for mentions of specific drugs, adverse events, or safety concerns. Natural language processing algorithms can identify relevant articles, extract key safety information, and prioritize content for human review.
Efficiency Gains: One pharmaceutical company reported that AI-powered literature monitoring reduced the time required for monthly literature reviews from 40 hours to 4 hours, while improving coverage by 300%.
AI also streamlines regulatory compliance by automating report generation and submission processes. These systems can automatically format safety data according to different regulatory requirements, generate periodic safety update reports (PSURs), and ensure timely submissions to global regulatory authorities.
Machine learning algorithms can learn from historical regulatory feedback, helping companies anticipate potential questions or concerns and prepare more comprehensive submissions.
A top-10 pharmaceutical company implemented an AI-driven pharmacovigilance platform across its global operations. The system processes over 200,000 adverse event reports annually and has achieved remarkable results:
The AI system integrates data from clinical trials, post-market surveillance, and literature sources, providing a comprehensive view of drug safety across the company’s portfolio.
A mid-sized biotech company specializing in oncology drugs faced challenges monitoring safety signals for their complex treatment regimens. Traditional methods struggled to identify interactions between multiple medications and patient-specific factors.
Their AI solution analyzes patient data, treatment protocols, and adverse event reports to identify safety patterns specific to different patient populations. The system has successfully identified several previously unknown drug interactions and enabled the company to provide more targeted safety guidance to healthcare providers.
A European regulatory authority partnered with technology companies to develop an AI system for analyzing adverse event reports across multiple pharmaceutical companies. The system:
This collaborative approach demonstrates how AI can improve safety monitoring at the industry level, benefiting patients regardless of which company manufactures their medications.
Successful AI implementation in pharmacovigilance requires careful planning and preparation. Companies must first assess their current capabilities, identify specific use cases, and develop a clear implementation strategy.
The AI pharmacovigilance market includes established technology vendors, specialized startups, and custom development options. Companies must evaluate potential partners based on several criteria:
Most successful AI implementations follow a phased approach, starting with pilot projects and gradually expanding scope:
Companies must establish clear metrics for evaluating AI implementation success:
Efficiency Metrics: Time savings, cost reductions, and productivity improvements Quality Metrics: Data accuracy, consistency, and compliance measures Strategic Metrics: Faster signal detection, improved risk management, and enhanced regulatory relationships
Regular monitoring and adjustment ensure AI systems continue delivering value as business needs evolve.
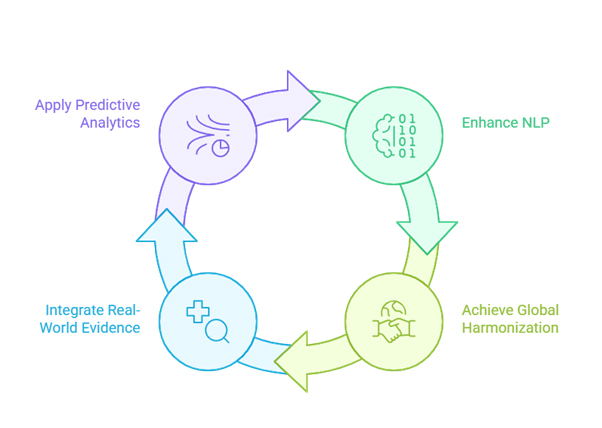
The next frontier in AI pharmacovigilance involves predictive analytics—using historical data and machine learning to anticipate future safety risks. These systems can identify patients at higher risk for adverse events, predict the likelihood of safety signals for new drugs, and recommend proactive risk mitigation strategies.
Emerging Capabilities: Advanced AI models are beginning to incorporate genomic data, patient lifestyle factors, and real-world evidence to create more personalized safety predictions. This personalized approach could revolutionize how companies assess and manage drug safety risks.
AI systems are increasingly incorporating real-world evidence from electronic health records, insurance claims, and patient registries. This broader data integration provides more comprehensive safety monitoring and enables detection of rare adverse events that might not appear in clinical trials.
Regulatory Support: Regulatory authorities are developing frameworks for using real-world evidence in drug safety assessments, creating opportunities for AI systems that can analyze diverse data sources.
AI has the potential to standardize pharmacovigilance practices across different regions and regulatory systems. Machine learning algorithms can adapt to different regulatory requirements while maintaining consistent safety analysis approaches.
Collaborative Opportunities: Industry consortiums and regulatory partnerships are exploring shared AI platforms that could improve safety monitoring for all stakeholders.
Next-generation AI systems will better understand context, nuance, and clinical reasoning in safety reports. These improvements will enable more accurate automated analysis and reduce the need for human review of routine cases.
Multilingual Capabilities: AI systems are becoming more sophisticated at processing safety reports in multiple languages, supporting global pharmacovigilance operations.
The integration of AI in pharmacovigilance represents a fundamental shift in how pharmaceutical companies approach drug safety. Leaders who embrace these technologies today position their organizations for success in an increasingly complex regulatory environment.
The integration of AI into pharmacovigilance is no longer a distant vision—it’s a present-day imperative. As predictive analytics, automation, and real-world data integration become more sophisticated, organizations that take proactive steps today will be better equipped to handle the demands of tomorrow. Embracing AI isn’t just about adopting new technology; it’s about transforming how safety data is collected, analyzed, and acted upon to protect patients more effectively.
By conducting a thorough evaluation of current processes, engaging with experts, and aligning AI strategies with regulatory expectations, pharmaceutical companies can unlock powerful efficiencies and insights. The future of pharmacovigilance is intelligent, responsive, and data-driven—and those who move early will lead the way in setting new standards for drug safety worldwide.