Know ALAT
ALAT-Assisted Literature Acquisition and Triage is a solution used for literature curation and review of published medical literature from various data sources.
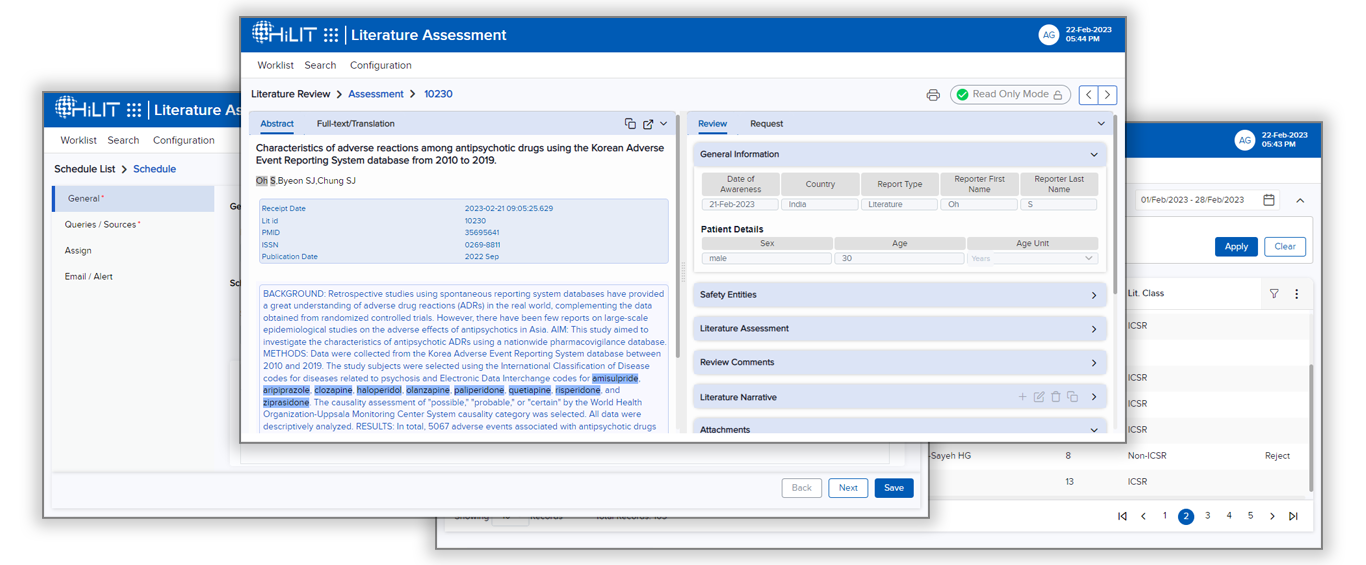
The ALAT system relies upon a robust machine learning AI system to extract key safety information like Product, Event, Patient Details, and Indications from the literature articles besides extracting other details.
The ALAT system is also integrated with the HiLIT platform’s case intake module to ease the workflow of reporting safety information to safety databases and regulatory reporting.