Overview
The vast majority of drug safety case processing and regulatory reporting applications are licensed, commercial-off-the-shelf product offerings. These systems provide many of the features and functions that are necessary to run the core business, however no out-of-the-box application is able to suit the specific needs of any particular organization. Not only has this, but requirements, regulations and technology evolved over time, prompting new product releases that require periodic system upgrades.
As a consequence of adopting a best-of-breed approach to application adoption, as well as strategically avoiding single product vendor dependence and lock-in, organizations tend to turn to system integrator to both implement the individual functional systems, but also to develop the necessary interfaces and integrations between these applications, to facilitate the end-to-end process flows. A simple example would be the transfer of adverse events from electronic data capture to the core safety systems and the transfer of source files, attachments from safety systems to centralized document repositories.
Vitrana meets these challenges by providing expert consulting services to implement drug safety case processing and reporting applications, developing key integrations to support cross-platform process flows, and maintaining and upgrading the platform, both for on premise or hosted deployment models. The Vitrana team are pioneers in cloud deployments and have worked with clients across the world who have realized the benefits of lower cost, of rapid implementation, and of closer alignment to the product road-map whilst ensuring the highest level of information security and data privacy.

Vitrana Team
Vitrana have designed Instructor Led Training courses that covers the basic and advanced concepts of managing drug safety systems including Argus for Global & Japanese users. The trainings have been designed to cater to requirements of different user types and categorized under following groups:
- End user training
- Advanced user training
- Business administrator training
- System administrator training

Business Configuration
Vitrana have invested in developing Gold configurations based on its vast experience of supporting global implementations. The Business Configuration phase entails maximization of reusability achieved from referencing to Vitrana’s Gold configurations and organization existing business configurations, validations assets, migration scripts and SOP’s. The approach ensures accelerated delivery and implementation of global best practices.
The consulting team have vast and extensive expertise on regulations and have lead numerous complex R3 Transition and IDMP programs.
The track entails the following activity set:
- Business Process Design
- User requirement and configuration specifications
- Configuration workshops
- Conference Room Pilots
- Configuration Builds
- SOP Updates
Data Migration
Vitrana Safety Consulting team has experienced bi-lingual resources with industry specific background and extensive experience of Data Migration across Safety Systems (ClinTrace, AERS, Argus, Perceive, Trackwise, Aster, Prodocumal).
The team have lead more than 15 Migration Programs involving large Global and Japanese Pharmaceutical organizations. The program complexity involves data from multiple source safety systems, existed in different timelines with intensive de-duplication & data merge.
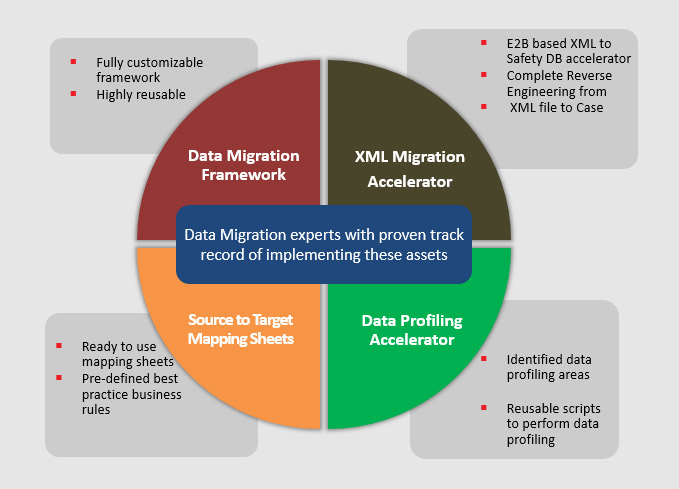
Vitrana have invested in developing a proprietary Data Migration & Integration Framework to accelerate complex migrations.
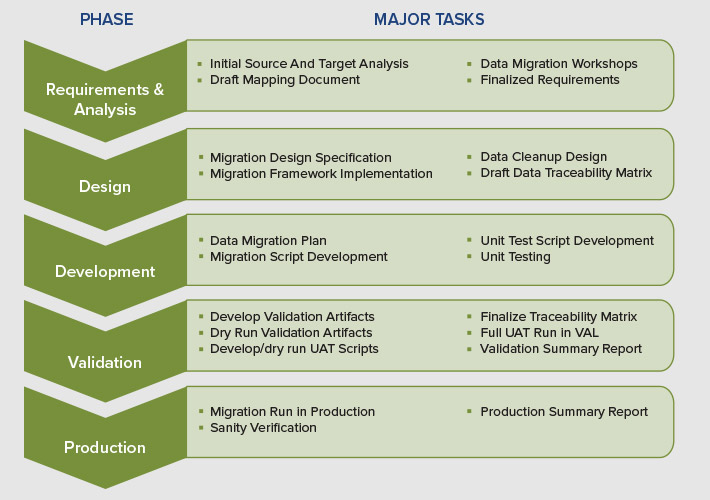
The SDLC lifecycle for a full lifecycle data migration program involves following phases:

Technology & Deployment
The service model entails the following key attributes:
- Database Administration and Optimization
- E2B gateway / pilot testing support
- E2B R3 BFC adapter and SME services
- Disaster Recovery Planning & Testing
- Cutover / Hypercare planning & rollout

Managed Services
Vitrana 24*7, follow the sun model with best in class infrastructure ensures delivery of application managed services for business critical safety systems. The team has bilingual capabilities with offshore operations ensuring highly cost effective services.
Build Your Team @ Vitrana (BYT @ Vitrana) offers a unique value preposition where customers can directly manage the team constitution and cost ensuring highest level of control and transparency. The service model entails the following key attributes:
- Issues, enhancements, or queries managed to completion
- Facilitate adherence to SLAs agreed with business
- End-to-end service management
Integration
Vitrana integration services portfolio has an extensive coverage of popular industry solutions including Argus, Medidata, Inform, Oracle Clinical, ONPOINT CTMS.
Vitrana Integration SME’s have delivered integrations between clinical and safety systems for 4 of the Top 10 Pharma
Vitrana have developed numerous accelerators to support integration among:

Reporting & Analytics
Vitrana Analytics Center of Excellence have delivered 300+ Japanese and English reports for drug safety organizations using all leading BI tools (OBIEE, Cognos, Business Objects, Spotfire and Tableau). Vitrana’s extensive exposure to multitude of Reporting and Analytics tools assist customers in choosing the right tool for the Reporting and Dashboard requirements.
Vitrana have invested in developing an open source reference warehouse model which can assist organizations to expedite the implementation program. The reporting and analytics services are categorized under following sub-groups:
- Mart/ETL Design & Development
- Analysis, Design & Development of custom reports
Validation
Vitrana Validation Center of Excellence have extensive experience in leading complex validation programs for safety implementation, upgrade, migration, reporting and integration. The team has bilingual (Japanese & English) capabilities. Many of its validation analysts have extensive experience of working as part of core product validation team including Argus ensuring deep insight into inter-related dependencies and coverage.
Vitrana have developed numerous accelerators to support expedited execution of validation phase. It has standardized validation packages to support Safety implementation & upgrade programs:
- Risk assessment, Validation strategy and planning
- Validation scripts authoring (IQ, OQ, PQ)
- Test execution & Summarization

Training
- End user training
- Advanced user training
- Business administrator training
- System administrator training
Vitrana Safety consulting team leverages its extensive Safety implementation & migration experience with multiple Tier 1 pharmaceutical companies, incorporating best practices approach into standard Oracle product to avoid unsupported customizations. The team bring value to an implementation project by use of accelerators increasing efficiency and reducing implementation efforts and cost.
