Intelligent Life Sciences Platform
supporting your complete Pharmacovigilance Operations
Pharmacovigilance Platform Modules
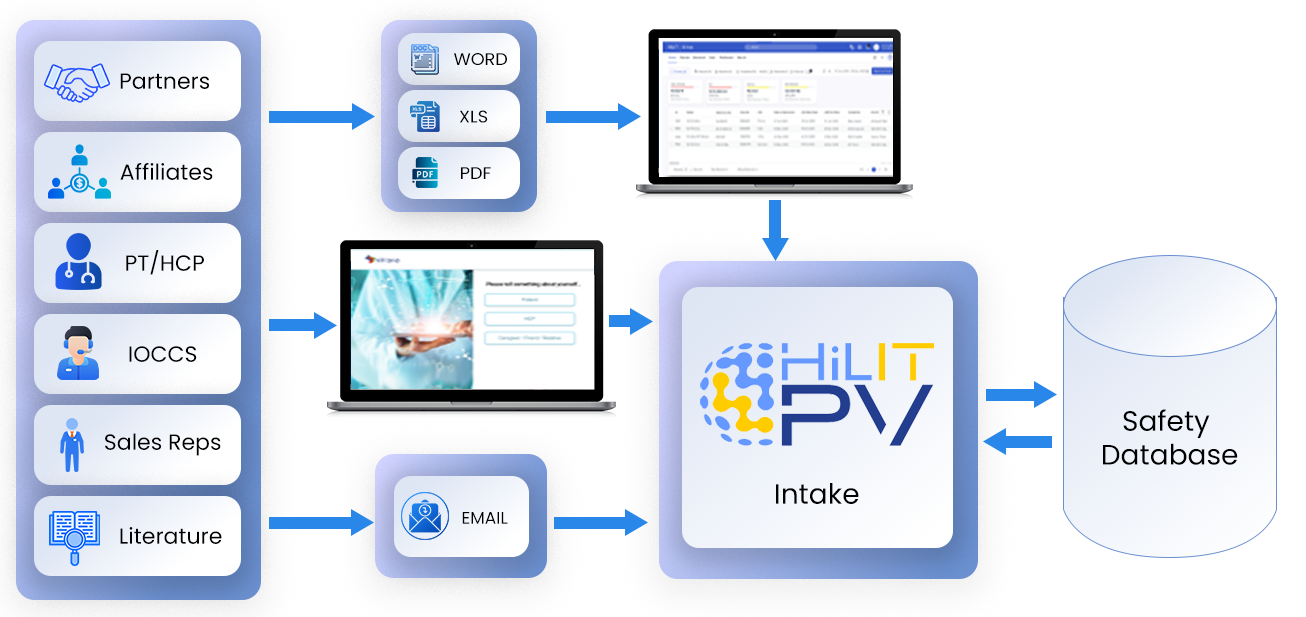
- Automated or on-demand monitoring and capturing from various data sources - PubMed, Medline, excel, etc.
- Assistive screening and scoring of literature articles, auto extraction of key elements (product, event, indication patient, reporter and COI)
- Auto-categorize / Re-categorize Article as ICSR / Non-ICSR
- Integration with Safety or Signal systems
- Multiple mailboxes with auto triage and classification
- Configurable forms with configurable validation rules for both external and internal web forms
- Workload management
- Collection from different sources
- External access via web portal
- Configurable forms and workflow
- Comprehensive QC
- Data Redaction
- Duplicate Search
- Multi-lingual Support
- Automated Case Export
- Structured Intake - CIOMS, MedWatch3500A
- Semi-structured Intake - SAE forms from PSPs, emails
- Unstructured Intake - Medical records, Literature
- Auto validations
- Auto translation
- Provide a Secured Mobile Intake in multiple languages
- Configured at the Application Level to use SSO
- Data Redaction
- Developed and deployed on iOS and Android
- Unified communication portal
- Intelligent Reminders
- Email agnostic
- Automated follow-up queries using configurable templates (via email or web links)
- System agnostic – integration with other intake sources, safety systems
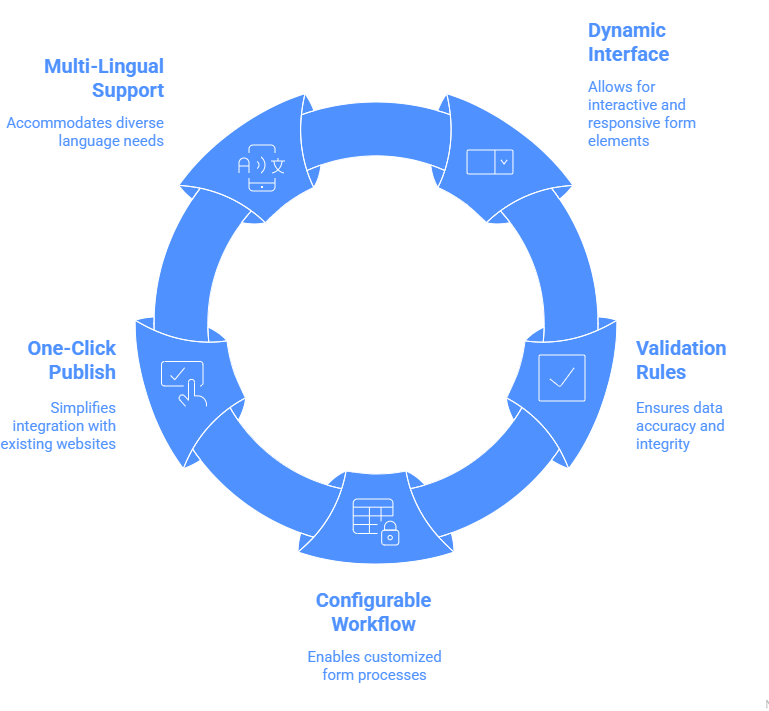
Unique Features
Supports current and future needs of Intake automation
Unique Features
Supports current and future needs of Intake automation
- Quickly design web forms with dynamic interface, validation rules and configurable workflow
- “One-click” publish capability to quickly integrate with existing web site / portal for AE collection
- Multi-lingual forms capable of supporting 30+ languages
- Rules based mailbox management system which can be configured to auto triage emails
- Centrally manage all PV email inboxes, including the ability to control access based on user, role and/or region
- OOB support for auto extraction from multiple formats including PDF, Word, Excel, XML, Emails
- Follow-up query management with survey forms capabilities
- Robust system and data access management capabilities providing flexibility to define controls and restrict access to support even complex global requirements
Data Flow
Case Study
Vitrana implemented HiLIT PV Intake in one of it’s customer servers (on-premise) which included:
- Web Forms
- Query Management
- Local submission tracking
- Mailbox management for Japan
- Provided local language support team in Japanese, Portuguese and Spanish
Results
Productivity
Data Quality
Conclusion
Vitrana’ s HiLIT PV Suite transformed the way business was conducted with tangible benefits to Productivity, Quality, Compliance and Cost to build a success story that led our client to be a market leader in the rare diseases' portfolio.
- Automated RSI document processing with extraction of datasheet elements and side-by-side comparison of Safety System vs. RSI calculated listedness
- Individual case or bulk case assessment (Excel upload) with direct RSI document viewing without external systems
- Flexible configuration at Product Family/Product/Indication levels with active window assessment comparing listedness within reporting period and RSI effective version
- Configurable complex logic for system-level and datasheet-level rules with auto-update or manual update of listedness in safety system
- Comprehensive audit trail for regulatory inspection readiness with sync capability from safety system (all/new/selected datasheets)
- Extract discrepancy reports for immediate action and version history tracking
- Reduces medical review time 80%+ and eliminates manual datasheet preparation
- Decoupled regional data entry from global workflow with support for Korea R3, China R3 (19 additional elements), Russia, LATAM countries - zero impact to global reporting timelines
- Three-step process: Automated extraction → Local affiliate transformation via worklist → Regional submission with complete audit trail
- Profile-based updates for current and future regional requirements with configurable validation rules for both ICH and regional elements - no global workflow changes needed
- Optional auto-translation module via external API with data push-back to safety database including full audit trail records
- Business maintenance capabilities: ACK file decoding, R3 batch consolidation, EVWeb auto-accept configuration, LATAM B3→C3 conversion
- Generate unique report outputs (EU MIR, PMDA cosmetics, custom regional formats) with automatic gateway submission
- Single solution supporting 10+ regional agencies with unified workflow used by 30+ organizations
Unique Features
- Automated identification of data errors with quality checks for both in-line case processing and end-of-line retrospective sampling
- "First Time Right" self-QC capability with individualized checklists, Medical Review QC module, and sample-based retrospective QC with statistical sampling
- Automated review with capability to capture RCA, action plans, and comments with complete audit trail of quality checks and findings
- Highly configurable to support organization-specific business requirements, validation rules, and case type variations
- Real-time quality metrics and performance tracking dashboards with drill-down to individual case details
- Integration with CAPA management for closed-loop quality issue follow-up and corrective action tracking
- Role-based access controls with export functionality for quality reports and regulatory compliance documentation
- Auto-identification of late cases and reports per configurable business SLAs with real-time tracking of individual and team performance against timelines
- Configurable logic for late calculation per business requirements (receipt date, awareness date, clock-stop rules) with comprehensive regional and product-specific visibility
- Automated alerts and notifications for cases approaching or exceeding deadlines with due-soon compliance line listings for proactive management
- Dashboard visualization of compliance trends by region, product, reporter type, and case type with drill-down from summary metrics to individual cases
- Historical trend analysis to identify systemic delays and process bottlenecks with configurable reminder schedules and escalation workflows
- Integration with case management systems for seamless workflow visibility and status tracking
- Export functionality for compliance reports, management presentations, and regulatory audit documentation
- Manage CAPA for PV process deviations and quality issues with automated alerts, reminders, and configurable email templates for milestone tracking
- Follow-up management workflow with status tracking (Open, In Progress, Under Review, Closed) and real-time monitoring of CAPA effectiveness and closure timelines
- Capture detailed RCA with predefined categories and free-text fields, link CAPAs to quality findings, late cases, audit observations, and deviation reports
- Action plan assignment with responsible parties, target completion dates, and document attachment capability for implementation evidence
- Comprehensive reporting on CAPA trends, categories, and closure rates with effectiveness verification tracking
- Integration with quality check modules for closed-loop quality management and continuous improvement feedback
- Audit-ready documentation with complete history of CAPA lifecycle from identification through effectiveness verification
- Query safety database using everyday business language with no technical expertise - type questions in natural language and retrieve results dynamically using advanced NLP and AI
- Supports complex queries handling joins, subqueries, and aggregations while processing user intent and natural variations in phrasing (e.g., "most side effects" = "top adverse reactions")
- Learns pharma-specific vocabulary using contextual prompts (e.g., DME matches Designated Medical Events) and searches inside free-text narratives and attachments (PDFs, Word docs)
- "Human-in-the-loop" feature with query review workflow allowing validation by technical teams before broader use with complete audit history for all executed queries
- Available in up to 39 languages including Japanese with results shown in tabular format and export to Excel/CSV/PDF
- Query library allows users to view, edit, or rerun previously submitted queries with secure role-based access for data confidentiality
- System interpretation review capability shows AI-generated SQL for technical validation and transparency
- Comprehensive PV-focused data mart with derived columns, tables, Facts and Dimensions optimized for reporting and analytics across all PV needs
- Support for various data sources including direct safety database integration (Argus, ARISg), sales data, Excel uploads and external systems
- Query Builder with configurable queries, auto-generation of case lists and option to run reports on saved lists for consistent analysis
- Seamless integration with any reporting and visualization tools (PowerBI, Tableau, Spotfire, Cognos) with out-of-box reports for operational management, compliance and aggregate reporting (PSUR, DSUR, PBRER)
- Historical data reporting with trend analysis, operational metrics dashboards and drill-down/drill-through capabilities with filtering and scheduling for automated distribution
- Extensible datamart model allowing addition of new data sources, metrics and regulatory intelligence tracker integration
- 21 CFR Part 11 compliant with complete user audit trail and role-based access controls for data security
Unique Features
Quadrant Suite - Integrated Quality, Compliance & Analytics Platform
- Interactive Web GUI based tool with built in access control for enhanced security
- Allows addition and update of Codelist including Flexi codelist and PLSR data
- Update of existing configuration data based on business key concept– Allows update of Japan specific fields
- Generation of Codelist and PLSR configuration data from Argus– Offline review and update of configuration data in excel
- Inbuilt exception handling and data validation– All Argus validations and rules built in to avoid loading of incorrect dataset
- Manage license linking updates (to meet Active Moiety requirements)– Automatic license link generation and upload as per organization’s active moiety requirements
- Support for Japanese fields– All J fields available in Codelist and PLSR supported
Transform Your Drug Safety Operations with AI-Powered Automation, Seamless Integration and Real-Time Compliance
The HiLIT platform revolutionizes pharmacovigilance with comprehensive modules covering critical processes in the safety life cycle.
From intelligent case intake and quality oversight to advanced reporting and regulatory submissions, our cloud-native solution reduces operational costs by 40% while ensuring 100% compliance across global markets.
Trusted by 8 of the top 10 pharmaceutical companies, HiLIT delivers predictive analytics, automated workflows, and seamless integration with existing systems like Argus, LSMV, Veeva, Oracle Analytics, Cognos Analytics and Power BI
Experience faster implementations, enhanced data quality and regulatory confidence with the industry's most comprehensive PV technology platform.
Intelligent case intake and data extraction with AI-powered automation Reduce manual processing by 50-75% across all data sources
Advanced case processing with automated workflows and quality checks Streamline medical review tasks and ensure consistent case assessment
Real-time monitoring and compliance dashboards for operational excellence Track KPIs, manage workloads, and maintain regulatory timelines
Automated regulatory submissions with global compliance Seamless submissions across ALL global agencies with
validation frameworks