
Overview
Today’s Life Sciences organizations face significant challenges in improving the quality of clinical services and meeting their regulatory compliance obligations. The success of life sciences organizations is predicated on many factors, but one that is particularly critical concerns the ability to fully leverage information assets to maximum effect. The proactive and efficient processing of real-time information across the enterprise can provide up-to-the-minute, analytical insights that help an organization to make effective and informed decisions. In addition, effective sharing of information across business applications enhances both internal efficiency and quality of service for internal stakeholders and customers alike. However, more often than not, these information assets lie hidden within the individual applications, preventing organizations from realizing their true potential.
Clinical & Safety Systems – Need for integration
- Clinical systems are Patient data driven system resulting in limited focus on capturing AE and Product information
- Drug safety system like Argus are AE data driven system which has Product and Event dependency
- Clinical systems have different templates per trial, hence there are different forms for each trial
- Argus has standardized case form and reporting across implementation
- Clinical systems perform the reporting based on the CDISC, Whereas Argus does the reporting based on ICH E2B(R2)
The Vitrana integration offering
The Vitrana Health Sciences integrated platform comprises a standards-based, integration service that is agnostic to the specific application product and related technology foundation, developing and re-using core adapters for various commonly adopted, COTS products across the healthcare and life sciences domains. The platform aims to deliver improvements in:
- data accessibility, transparency and visibility
- operational efficiency, productivity and clinical outcomes
- collaboration among research and patient care entities
- identification of signals based on real-time events
- data quality and access controls
- total cost of IT ownership through re-use of integration technologies
- regulatory compliance, including reporting
- end user/patient experience
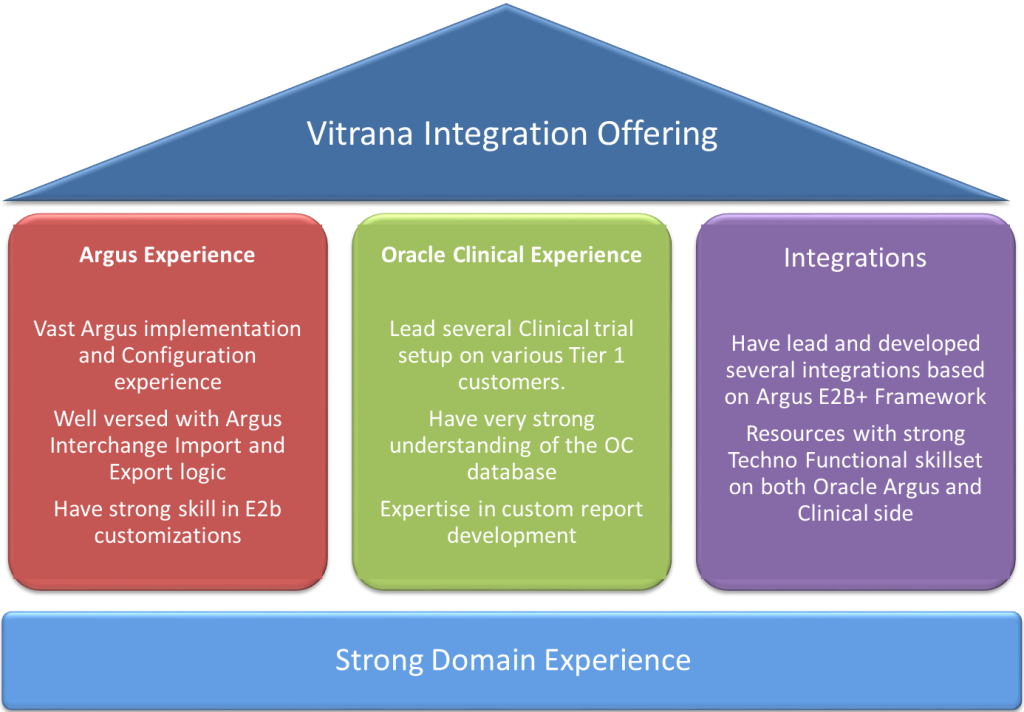
Our Integration Experience
Our team has lead and developed integration solutions for Argus using E2B+ at several Tier 1 pharmaceutical firms based out of US and Europe
- Outgoing integration with Product Quality Complaints System
- Bidirectional integration with Clinical EDC systems
- Integration with Japan based system
Vitrana has a wide portfolio of key integration offerings for Oracle Health Sciences products that include:
- Argus Safety and InForm
- Argus Safety and Medidata Rave
- Argus J and Common Master Database (CMDB)
- Argus Safety with Siebel
- CTMS with e TMF solutions
- CTMS and Financial Applications
- CTMS with EDC/CDMS applications
- Clinical, PV and Healthcare Data Warehouse
